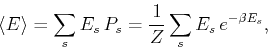Most physical systems are not isolated, bu exchange energy with the
environment. Since the system is very small compared to the environment,
we consider that the environment acts effectively as a heat reservoir or
heat bath at a fixed temperature ![]() . If a small system is put in
thermal contact with the heat bath, it will reach thermal equilibrium
exchanging energy until the system attains the temperature of the bath.
. If a small system is put in
thermal contact with the heat bath, it will reach thermal equilibrium
exchanging energy until the system attains the temperature of the bath.
Imagine an infinitely large number of mental copies of the system and the
heat bath. The probability ![]() that the system is found in a microstate
that the system is found in a microstate
![]() with energy
with energy ![]() is given by:
is given by:
We can use (276) to obtain the ensemble average of physical
quantities of interest. For instance, the mean energy is given by: