


Next: Limitations of the tight-binding
Up: General case: Linear Combination
Previous: Example 5.1: Single s
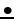 The characteristic feature of the tight-binding energy bands is that hte bandwidth is determined by the small overlap integral
The characteristic feature of the tight-binding energy bands is that hte bandwidth is determined by the small overlap integral 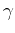 . Thus, the tight-binsing bands are narrow bands, and the smaller the overlap, the narrower the bands are. In the limit of vanishing overlap, the bandwidth also vanishes and the states become
. Thus, the tight-binsing bands are narrow bands, and the smaller the overlap, the narrower the bands are. In the limit of vanishing overlap, the bandwidth also vanishes and the states become 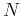 -fold degenerate. This would correspond to core electrond residing near the nucleus, resembling
-fold degenerate. This would correspond to core electrond residing near the nucleus, resembling 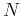 isolated atoms, or atoms that are pulled very far apart.
isolated atoms, or atoms that are pulled very far apart.
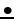 Interestingly, although commonly associated to the kinetic energy, the integral
Interestingly, although commonly associated to the kinetic energy, the integral 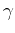 -also called hopping integral- is purely generated by the potential energy, and how it hybridizes neighboring orbitals.
-also called hopping integral- is purely generated by the potential energy, and how it hybridizes neighboring orbitals.
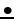 Near the bottom of the bands, the energy is quadratic in
Near the bottom of the bands, the energy is quadratic in 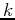 , and the constant-energy surfaces are spherical.
, and the constant-energy surfaces are spherical.
 The slope of the energy curve is zero when crossing perpendicular to one of the faces of the Brillouin zone.
The slope of the energy curve is zero when crossing perpendicular to one of the faces of the Brillouin zone.
 In solids that are not monoatomic Bravais lattices, i.e they are decorated lattices with more than one atom species, the tight-binding calculation becomes more complicated. if we have more than one atom per unit cell, we can write:
In solids that are not monoatomic Bravais lattices, i.e they are decorated lattices with more than one atom species, the tight-binding calculation becomes more complicated. if we have more than one atom per unit cell, we can write:
 |
(204) |
where  denotes the different atoms in the unit cell. Then, we need to generalize the equations to obtain the matrix elements
denotes the different atoms in the unit cell. Then, we need to generalize the equations to obtain the matrix elements
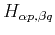 , and
, and
 .
.



Next: Limitations of the tight-binding
Up: General case: Linear Combination
Previous: Example 5.1: Single s
Adrian E. Feiguin
2009-11-04
![]() Interestingly, although commonly associated to the kinetic energy, the integral
Interestingly, although commonly associated to the kinetic energy, the integral ![]() -also called hopping integral- is purely generated by the potential energy, and how it hybridizes neighboring orbitals.
-also called hopping integral- is purely generated by the potential energy, and how it hybridizes neighboring orbitals.
![]() Near the bottom of the bands, the energy is quadratic in
Near the bottom of the bands, the energy is quadratic in ![]() , and the constant-energy surfaces are spherical.
, and the constant-energy surfaces are spherical.
![]() The slope of the energy curve is zero when crossing perpendicular to one of the faces of the Brillouin zone.
The slope of the energy curve is zero when crossing perpendicular to one of the faces of the Brillouin zone.
![]() In solids that are not monoatomic Bravais lattices, i.e they are decorated lattices with more than one atom species, the tight-binding calculation becomes more complicated. if we have more than one atom per unit cell, we can write:
In solids that are not monoatomic Bravais lattices, i.e they are decorated lattices with more than one atom species, the tight-binding calculation becomes more complicated. if we have more than one atom per unit cell, we can write:
